The Kiernicki Lab is a multi-disciplinary group of researchers that takes pride in our synthetic prowess. We utilize synthetic approaches to address challenging problems in science. Our interests range from simple coordination chemistry to biomimetics, from methods to minimize our fossil fuel reliance to alternative approaches for wastewater remediation, and everything in between. Ongoing research projects are listed below. If you have questions about any of these projects or are interested in designing your own project, contact Professor Kiernicki!
Project 1
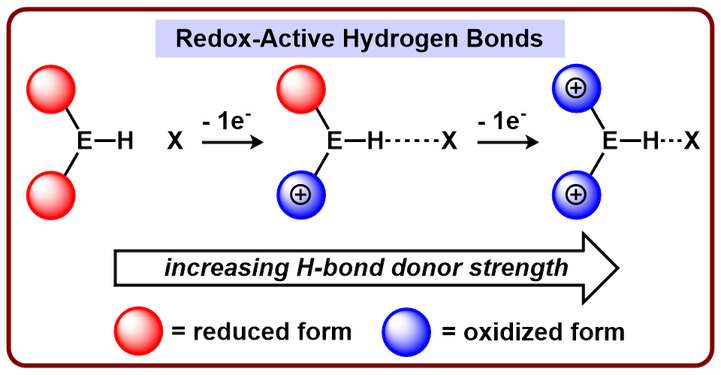
Redox Active Hydrogen Bonds: a New Method for Unparalleled Reaction Control
Hydrogen bonding interactions are omnipresent in the biological world and are now appreciated by synthetic chemists as an invaluable tool for controlling reaction energetics. A key advantage that biology possesses is the ease with which hydrogen bond donor/acceptor strength can be modified through structural reorganization, allostery, and/or additional acidic/basic interactions. Our lab is interested in methods to control hydrogen bond strength in situ within a reaction flask. Currently, we are exploring the utility of adjacent redox-active centers to influence hydrogen bond donor/acceptor strength.
Project 2
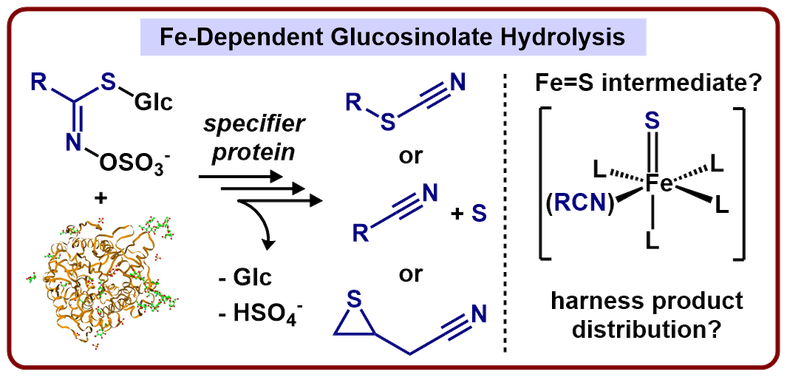
An Inorganic Probe of a Biochemical Mystery
Our laboratory has a long-standing interest in understanding the unique bond activation reactions and resulting product specificity observed in biological systems. A recent nutritional fad that emphasizes glucosinolate-rich diets has led us to question why consumption of plants from the family Brassicaceae may lead to improved health. During glucosinolate hydrolysis, iron-containing specifier proteins are capable of intercepting aglucone intermediates and affecting their decomposition pathway. Our lab has become interested in the molecular scale description of these iron-containing specifier proteins and, specifically, what role they play mechanistically in glucosinolate hydrolysis product distribution.
Project 3
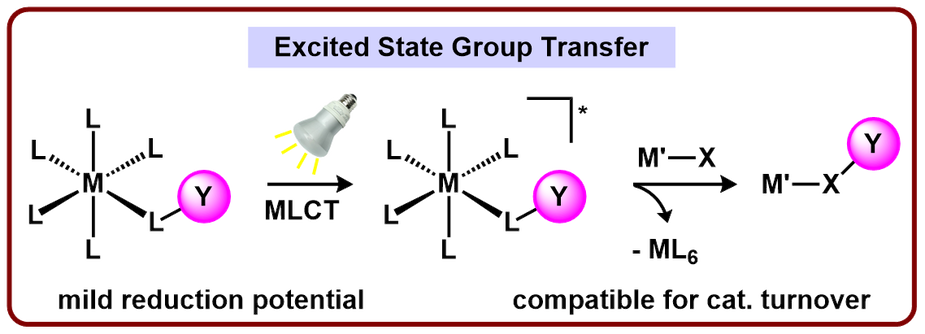
A Photochemical Approach to Upgrading Chemical Feedstocks
A common goal of our lab is to develop methods to perform challenging chemical transformations more efficiently. While routes to cleave/functionalize extremely strong bonds (e.g. C≡O, M=O) are known, they often rely on extraordinarily harsh reaction conditions to overcome daunting redox requirements. We are targeting mild methods to circumvent these harsh conditions, and, ultimately, design systems that are compatible for catalytic turnover.